The FDA’s recent approval of subcutaneous pembrolizumab (Keytruda Qlex) for solid tumors means that mesothelioma patients will have a more convenient treatment option available to them. Subcutaneous treatments can be administered quickly under the skin rather than through time-consuming intravenous infusions. The approval covers all types of solid tumor cancers for which intravenous pembrolizumab has already been approved.
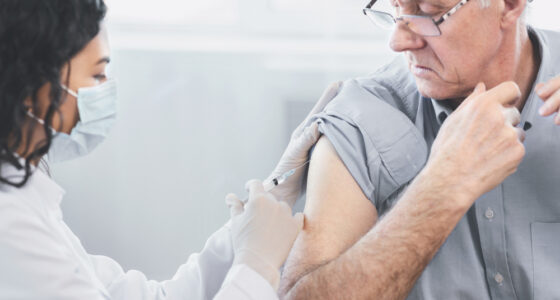
Mesothelioma Qualifies for New Treatment Approach
Mesothelioma is a rare form of cancer caused by exposure to asbestos. Unlike blood cancers that circulate throughout the body, it is made up of tumors in the mesothelial tissues that line the lungs, abdomen, or heart. The disease starts when the toxic mineral’s fibers damage the cells of this lining, leading to tumor formation that appears as solid masses on CT scans and other imaging tests, and which can spread.
Previously, mesothelioma patients could only receive the immunotherapy drug pembrolizumab through intravenous administration: With the new approval, they can now receive it through a simple injection under the skin that will take minutes rather than hours.
FDA Approval Follows Successful Tests on Lung Cancer Tumors
Mesothelioma patients who choose the new subcutaneous treatment can expect it to have a safety and effectiveness profile similar to the IV version. In the study that drove the approval, lung cancer patients receiving the new approach showed response rates of 45% compared to 42% for those receiving IV treatment. Survival outcomes were essentially identical between the two methods, with no meaningful differences in how long patients lived or how long their tumors remained controlled.
The most common side effects in the study included anemia, low white blood cell counts, nausea, and fatigue—effects typically associated with the combination cancer treatment rather than the injection method itself. Only 2.4% of patients experienced injection-site reactions, typically involving mild redness or discomfort. Most patients tolerated the treatment well, with discontinuation rates lower than those of patients who received the treatment via IV. administration.
This approval is a significant quality-of-life improvement for mesothelioma patients, making treatment more accessible and convenient. For information on other breakthroughs and resources, contact the Patient Advocates at Mesothelioma.net today at 1-800-692-8608.
